Have you ever stopped to really think about the delightful smells around you, perhaps the sweet aroma of a ripe apple, or maybe the fresh, clean scent of a newly laundered shirt? Well, quite often, the secret behind these pleasant smells, and indeed many other fascinating properties, comes down to a special kind of chemical compound. This is where the world of ester ana truly opens up, offering a glimpse into some very common yet incredibly versatile molecules that play a big part in our daily lives.
It's fascinating, really, how these tiny building blocks of matter can create such a wide array of experiences for us, isn't it? From the vibrant flavors that make our food enjoyable to the subtle notes in our favorite perfumes, esters are, in a way, the unsung heroes of sensory delight. They are quite literally everywhere once you start looking for them, and understanding what they are can really change how you see the things you use and enjoy every single day.
So, perhaps you're curious about what makes these compounds so special, or how they come to be. Or maybe you're just wondering why they're so important in industries like food and cosmetics. We're going to explore what an ester is, how it's put together, and why it's such a significant player in the chemical world, giving you, you know, a pretty good idea of its foundational role.
Table of Contents
- Understanding Ester Ana: A Chemical Profile
- How Esters Are Formed and Named
- The Many Roles of Ester Ana in Our World
- When Esters Change: Hydrolysis and Saponification
- Common Questions About Esters
- Conclusion: The Pervasive Presence of Ester Ana
Understanding Ester Ana: A Chemical Profile
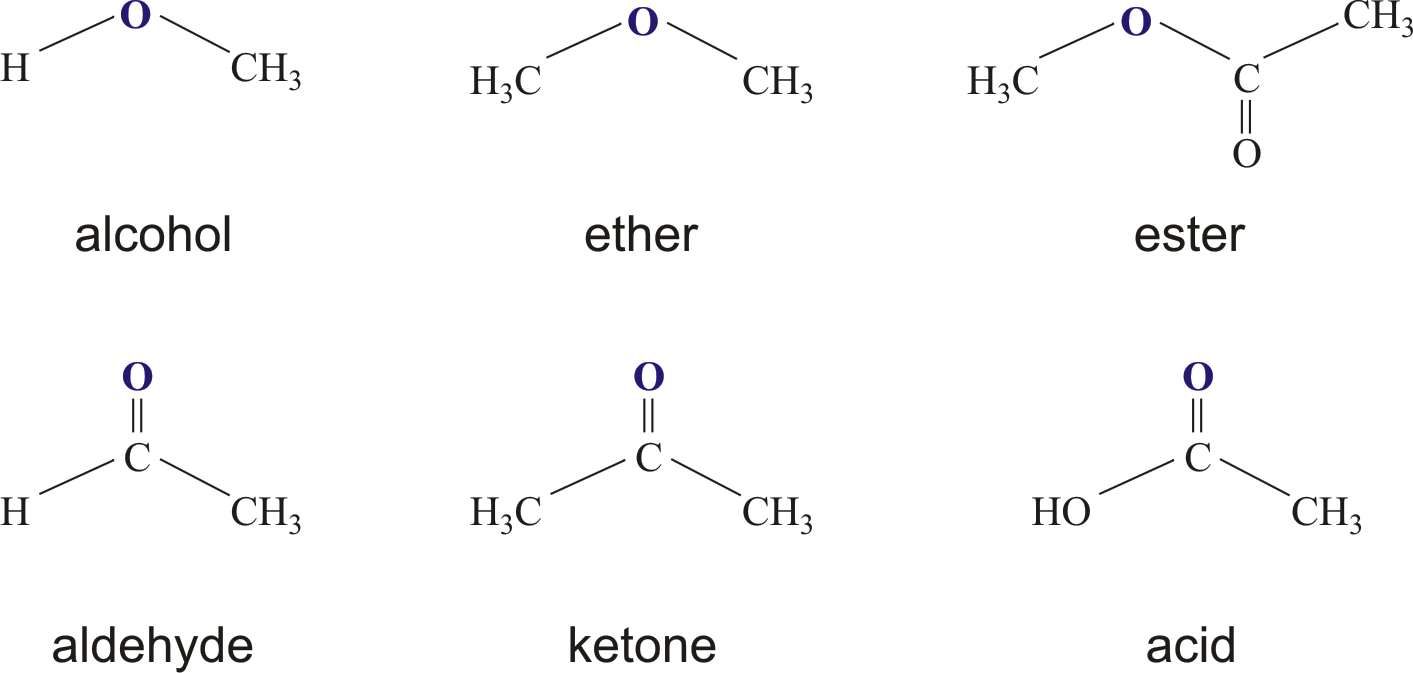
When we talk about `ester ana`, we're really focusing on a class of organic compounds known simply as esters. From a chemistry point of view, an ester is a compound that comes from an acid, whether that acid is organic or inorganic. What happens is that the hydrogen atom, usually found in at least one acidic hydroxyl group of that acid, gets replaced by another group, typically a hydrocarbon group. It's a pretty neat trick, actually, how these molecules are put together.
These compounds are, you know, quite distinct. They're a class of organic compounds that have a particular way of reacting with water. When they meet water, they produce alcohols and either organic or inorganic acids. This reaction is a pretty important one in chemistry, giving us a way to break down these compounds into their original parts. Carboxylic esters, derived from carboxylic acids, are the most common type you'll encounter, and they are, in some respects, the stars of this chemical family.
An ester is, basically, an organic compound where the hydrogen in the compound's carboxyl group gets replaced with a hydrocarbon group. This unique structure is what gives esters their specific properties and their wide range of applications. It's a functional group that's derived from the condensation of an alcohol and an acid, and this process happens with a simultaneous loss of water. So, it's a bit like two pieces coming together and shedding a bit of themselves to form something new.
Key Characteristics of Esters
Here are some key characteristics that help us understand esters a bit better, sort of like their vital statistics:
- Origin: Derived from acids (organic or inorganic) where a hydrogen atom from an acidic hydroxyl group is replaced by a hydrocarbon group.
- Common Type: Carboxylic esters are the most frequently encountered, coming from carboxylic acids.
- Functional Group: Often referred to as a carboxylate ester, formed through the condensation of an alcohol and an acid.
- Structural Feature: In an ester, the second oxygen atom bonds to another carbon atom, which is a very defining part of its structure.
- Reactivity with Water: They react with water to yield alcohols and acids.
- Naming Convention: Names include prefixes that show the lengths of the carbon chains within the molecules.
How Esters Are Formed and Named
Understanding how esters are created and how we give them their names helps us, you know, make sense of their chemical identity. It's a bit like learning the grammar of their molecular language. The process of making an ester is quite specific, and it involves some pretty common chemical players.
The Building Blocks of Esters
Esters are, in essence, built from two main components: an acid and an alcohol. Imagine, for instance, ethanoic acid and ethanol. When these two substances come together under the right conditions, they react. The diagram, if you were to see one, would show the relationship between the ethanoic acid, the ethanol, and the resulting ester. It's a kind of chemical dance where parts are swapped, and a new compound is formed, and that new compound is the ester.
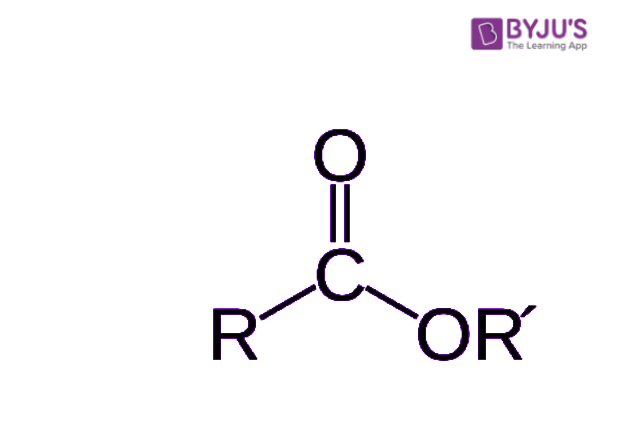
This process is often called esterification, and it's a very important reaction in organic chemistry. It's how many of these compounds, which are used for so many different things, are actually produced. So, really, it's a fundamental concept to grasp when you're looking at how these compounds come into being. The general structure for an ester is something you can learn to identify, which is quite helpful for chemists.
Naming These Compounds
When it comes to naming esters, there are a couple of systems we use. There are common names, which are often simpler and perhaps more familiar to those outside of chemistry circles. Then there's the IUPAC system, which is the international standard for naming chemical compounds. This system provides a very systematic way to name esters, ensuring that chemists all over the world can, you know, understand exactly which molecule is being discussed.
The names for esters typically include prefixes that denote the lengths of the carbon chains in the molecules. This is a very useful piece of information, as it tells you a lot about the size and, in some respects, the shape of the ester molecule. For example, if you see "ethyl acetate," the "ethyl" part tells you about one of the carbon chains, and "acetate" tells you about the other. It's a bit like giving a first and last name to the molecule, so to speak.
The Many Roles of Ester Ana in Our World
The chemical family of `ester ana` truly shines in its practical applications, touching our lives in ways we might not even realize. Their unique properties make them incredibly valuable, particularly in areas where pleasant smells and tastes are highly sought after. It's pretty amazing, really, how a single type of compound can have such a wide impact.
Flavors and Fragrances: The Sweet Side of Chemistry
One of the most prominent uses for esters is, honestly, in the flavor and fragrance industry. Think about the sweet, fruity smell of bananas, pineapples, or apples. Many of these natural aromas are due to the presence of specific esters. For example, isoamyl acetate gives bananas their characteristic smell, while methyl salicylate is responsible for the scent of wintergreen. They are, in a way, the essence of these delightful sensory experiences.
Because of their pleasant and often distinctive odors, esters are widely used in perfumes, colognes, and air fresheners. They also find their way into food products as artificial flavorings, making candies taste like strawberries or drinks like peaches. It's a very clever use of chemistry to enhance our everyday enjoyment, and it's pretty much everywhere you look, or rather, smell.
Beyond the Smell: Other Practical Uses
While their role in scents and tastes is perhaps the most famous, esters have other important applications too. For instance, some esters are used as solvents in paints, varnishes, and lacquers. Their ability to dissolve other substances makes them quite useful in these formulations. They are, you know, good at helping things mix and spread evenly.
Moreover, esters can be found in plastics and polymers. For example, polyesters are a broad class of polymers that contain ester linkages in their main chain. These materials are used to make everything from clothing fibers to plastic bottles. So, they are, in some respects, the backbone of many modern materials we rely on daily. It's quite a versatile group of compounds, actually.
When Esters Change: Hydrolysis and Saponification
Just as esters are formed, they can also be broken down, and this process is just as important in chemistry and industry. Understanding how esters react, especially with water, helps us appreciate their dynamic nature. It's a pretty fundamental aspect of their chemical behavior, you know, how they can transform.
Breaking Down Esters
An ester can be hydrolyzed, which basically means it reacts with water. This can happen either by using an aqueous base or an aqueous acid. When this reaction occurs, the ester yields a carboxylic acid plus an alcohol. This is, in a way, the reverse of the esterification process we discussed earlier. It's a method to get back the original components that made the ester in the first place, which can be very useful in various chemical processes.
This breaking down process is, you know, quite important for recycling chemicals or for understanding how certain compounds degrade in nature. It shows that these molecules are not static but can change and transform under the right conditions. It's a testament to the dynamic nature of chemical reactions, really.
Saponification: The Soap-Making Connection
A very specific and historically significant type of ester hydrolysis is called saponification. This term comes from the Latin word "sapo," which means soap. Ester hydrolysis in a basic solution is precisely what saponification is. This reaction is how soaps are traditionally made, by reacting fats (which are essentially long-chain esters) with a strong base like lye. The result is soap and glycerol.
So, the next time you wash your hands with a bar of soap, you're actually witnessing a chemical reaction involving ester hydrolysis. It's a pretty cool connection between fundamental chemistry and an everyday product. This process is, you know, a classic example of how understanding chemical reactions can lead to practical and widely used inventions.
Common Questions About Esters
People often have questions about esters, especially since they're so common in our lives. Here are a few things folks often wonder about these interesting compounds:
What is the main use of esters?
Well, the primary use of esters is, very often, in the flavor and fragrance industry. They're responsible for many of the pleasant smells and tastes we encounter in fruits, perfumes, and artificial flavorings. It's pretty much their star role, you know, making things smell and taste good.
How are esters formed?
Esters are typically formed through a reaction called esterification. This happens when an alcohol reacts with an acid, with the simultaneous loss of a water molecule. It's a condensation reaction, basically, where two molecules join and a smaller molecule, water, is removed.
Are esters good for anything?
Absolutely! Esters are incredibly useful. Besides their role in flavors and fragrances, they're also used as solvents, in the production of plastics like polyesters, and even in the making of soaps through saponification. So, yes, they are, you know, quite versatile and beneficial in many ways.
Conclusion: The Pervasive Presence of Ester Ana
So, we've taken a little journey into the world of `ester ana`, understanding that this term points to the fascinating chemical compounds known as esters. We've seen how they are formed from acids and alcohols, and how their unique structure gives them their distinct properties. From the sweet scents that fill our homes to the flavors that make our food enjoyable, esters are, in some respects, truly everywhere.
Their ability to be created and broken down, as seen in hydrolysis and saponification, highlights their dynamic nature and their importance in various industrial processes. It's pretty clear that these compounds, while often unseen, play a very significant role in the chemistry that shapes our daily experiences. To learn more about organic chemistry on our site, you can, you know, explore other related topics. And if you're curious about the specific reactions, you can always check out this page on ester chemistry for additional details.